Call us now :+8613788933411
Voxilaprevir chemical
Voxilaprevir chemical Specification
- Molecular Formula
- C40H52F4N6O9S
- Storage
- Freezer
- Molecular Weight
- 868.93 Kilograms (kg)
- Place of Origin
- China
- Other Names
- GS9857;Voxilaprevir
- CAS No
- 1535212-07-7
- Grade
- Medicine Grade
- Usage
- Voxilaprevir is a hepatitis C virus (HCV) nonstructural (NS) protein 3/4A protease inhibitor that is used in combination with sofosbuvir and velpatasvir.
- Purity
- 99%
- Appearance
- powdr
- Smell
- Other
- Color
- Other
- Form
- Powder
Voxilaprevir chemical Trade Information
- Minimum Order Quantity
- 1 Ton
- FOB Port
- Shanghai
- Payment Terms
- Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Delivery Point (DP), Cash Against Delivery (CAD), Telegraphic Transfer (T/T), Days after Acceptance (DA)
- Supply Ability
- 1000 Tons Per Week
- Delivery Time
- 10 Days
- Sample Available
- Yes
- Sample Policy
- Contact us for information regarding our sample policy
- Main Export Market(s)
- Australia, Eastern Europe, Western Europe, Middle East, Africa, Central America, South America, Asia, North America
About Voxilaprevir chemical
1R2RN1R2R255difluoro53hydroxy6methoxy2quinoxalinylpentylcyclopropyloxycarbonyl3methylLvalyl3S4R3Ethyl4hydroxyLprolyl1amino2difluoromethylN1 methylcyclopropylsulfonylcyclopropanecarboxamide cyclic 1 2ether
CAS NO 1535212077
Molecular Formula C40H52F4N6O9S
Molecular Weight 86893
Potent Hepatitis C Virus Treatment
Voxilaprevir acts by inhibiting the NS3/4A protease, a crucial protein for hepatitis C virus replication. When used alongside sofosbuvir and velpatasvir, it enhances the therapeutic regimen against HCV, offering a robust option for patients seeking effective viral clearance. Its high purity and specialized storage requirements ensure consistent performance in clinical settings.
Pharmaceutical Grade and Quality Assurance
Manufactured under strict standards in China, Voxilaprevir is supplied with a purity of 99%. This guarantees reliable integration into medical formulations. As a medicine grade product, it meets the highest industry benchmarks for safety, efficacy and quality, ensuring optimal results for end users and healthcare professionals.
FAQs of Voxilaprevir chemical:
Q: How should Voxilaprevir be stored to maintain its efficacy?
A: Voxilaprevir should be stored in a freezer to ensure its stability and preserve its potency. Maintaining the appropriate storage conditions helps retain its effectiveness in pharmaceutical applications.Q: What is the primary usage of Voxilaprevir in medicine?
A: Voxilaprevir is primarily used as a hepatitis C virus (HCV) NS3/4A protease inhibitor. It forms part of a combination therapy with sofosbuvir and velpatasvir for treating chronic HCV infections.Q: When is Voxilaprevir typically administered in the clinical setting?
A: Voxilaprevir is administered as part of a prescribed antiviral regimen for patients diagnosed with hepatitis C. Timing and dosage depend on medical guidelines and patient-specific factors assessed by healthcare professionals.Q: Where is Voxilaprevir manufactured and supplied from?
A: Voxilaprevir is manufactured and supplied from China, where pharmaceutical companies specialize in the production, export, and distribution of high-grade chemical ingredients.Q: What is the process of using Voxilaprevir in combination therapy?
A: In clinical practice, Voxilaprevir is combined with sofosbuvir and velpatasvir to enhance treatment efficacy against HCV. This multi-drug approach is designed to target multiple viral proteins and increase the likelihood of successful outcomes.Q: What are the benefits of using Voxilaprevir in HCV treatment protocols?
A: Using Voxilaprevir provides potent inhibition of a key viral protein, improving viral clearance and treatment success rates when used in combination therapies. Its high purity ensures consistent results and patient safety.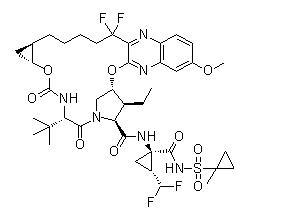

Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Chemicals Category
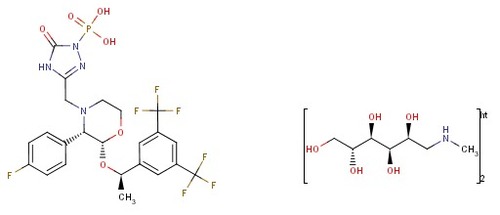
fosaprepitant dimeglumine
Minimum Order Quantity : 1 Ton
CAS No : 265121048
Grade : Medicine Grade
Application : Pharmaceutical Industry
Molecular Formula : C60H78F14N10O20P2
Molecular Weight : 1587.2414868 Grams (g)
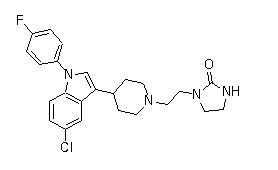
Sertindole chemical
Minimum Order Quantity : 1 Ton
CAS No : 106516249
Molecular Formula : C24H26ClFN4O
Molecular Weight : 440.94 Drams (dr)
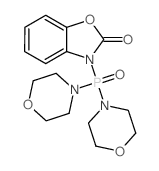
3-dimorpholin-4-ylphosphoryl-1 3-benzoxazol-2-one
Minimum Order Quantity : 1 Ton
CAS No : 54349950
Grade : Medicine Grade
Application : Pharmaceutical Industry
Molecular Formula : C15H20N3O5P
Alvimopan dihydrate
Minimum Order Quantity : 1 , , Ton
CAS No : 170098381
Grade : Medicine Grade
Application : Pharmaceutical Industry
Molecular Formula : C25H32N2O4.2(H2O)
Molecular Weight : 460.57 GSM (gm/2)

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry