Call us now :+8613788933411
Tiaprost chemicals
MOQ : 1 Ton
Tiaprost chemicals Specification
- Molecular Weight
- 396.5 Grams (g)
- Storage
- Freezer
- Place of Origin
- China
- Molecular Formula
- C20H28O6S
- Other Names
- Tiaprost;7-[3,5-Dihydroxy-2-[3-hydroxy-4-(3-thienyloxy)-1-butenyl]cyclopentyl]-5-heptenoic acid
- CAS No
- 71116-82-0
- Grade
- Medicine Grade
- Purity
- 99%min
- Appearance
- solid
- Application
- Pharmaceutical Industry
- Form
- Solid
Tiaprost chemicals Trade Information
- Minimum Order Quantity
- 1 Ton
- FOB Port
- Shanghai
- Payment Terms
- Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Paypal, Telegraphic Transfer (T/T)
- Supply Ability
- 1000 Tons Per Week
- Delivery Time
- 10 Days
- Sample Available
- Yes
- Sample Policy
- Contact us for information regarding our sample policy
- Packaging Details
- foil/bag/bottle
- Main Export Market(s)
- Eastern Europe, Western Europe, Africa, Central America, Middle East, South America, Asia, North America, Australia
About Tiaprost chemicals
We offer Tiaprostwith the following product specification:
Features:
- High purity
- Free sample available
- Lower price
- Experienced service team
- Fast delivery
- Considerate after-sale service
Looking for the ideal CAS no.71116-82-0 manufacturer & supplier? we are in the occupation of providing a superior quality range ofTiaprostto our most valued client. Each product are professionally under sophisticated detection equipment of a reliable quality assurance. We are China origin factory of High purityTiaprost. If you have any question, please dont hesitate to contact us.
- CAS NO.: 71116-82-0
- Molecular Formula: C20H28O6S
- Formula Weight: 396.5
Superior Medicine Grade Purity
Tiaprost is crafted to exceed pharmaceutical industry requirements, boasting a remarkable purity of 99% minimum. This high standard ensures its suitability for sensitive medical applications. Its reliable consistency and certified composition make it a trusted ingredient in sophisticated drug formulations, strictly controlled through comprehensive quality protocols.
Optimal Storage & Handling
To guarantee maximum stability and extend shelf life, Tiaprost must be stored in a freezer. This cold storage condition preserves its chemical integrity, preventing degradation and maintaining potency for pharmaceutical use. Proper handling and storage are essential to uphold the effectiveness and safety profile of this solid chemical compound.
Manufactured and Supplied for Global Pharmaceutical Markets
Originating from China, Tiaprost is available through an extensive network of manufacturers, exporters, suppliers, and traders, especially for the Indian pharmaceutical market. This broad distribution framework ensures timely availability and maintains the products quality from production to delivery, meeting demands of major healthcare industries worldwide.
FAQs of Tiaprost chemicals:
Q: How should Tiaprost be stored for maximum stability?
A: Tiaprost must be kept in a freezer to preserve its chemical stability and prevent any degradation, ensuring it maintains its effectiveness for pharmaceutical applications.Q: What is the primary use of Tiaprost in the pharmaceutical industry?
A: Tiaprost is primarily utilized as a high-purity active pharmaceutical ingredient for the development and manufacturing of specialized medications, owing to its reliable medicinal grade and specific chemical properties.Q: When is Tiaprost typically incorporated into pharmaceutical formulations?
A: Tiaprost is integrated during the formulation or synthesis phases of drug development, particularly when a highly pure, effective compound is necessary for targeted therapeutic drugs.Q: Where does Tiaprost originate and how is it distributed?
A: Tiaprost is manufactured in China and supplied globally, including India, through an established network of distributors, exporters, and suppliers focused on serving the pharmaceutical sector.Q: What is the process involved in handling Tiaprost safely?
A: Handling Tiaprost requires adherence to pharmaceutical safety standards, including the use of gloves, protective clothing, and strict temperature control, especially keeping the product frozen during storage and transport.Q: What are the key benefits of using Tiaprost with 99% purity?
A: The high purity (minimum 99%) of Tiaprost ensures minimal impurities, translating into greater efficacy and safety for end pharmaceutical products. This consistency supports precise dosing and reliable therapeutic outcomes.Q: How can Tiaprost be obtained for pharmaceutical manufacturing in India?
A: Pharmaceutical companies in India can acquire Tiaprost from authorized distributors, exporters, and manufacturers who specialize in medicine-grade chemicals, ensuring regulatory compliance and quality assurance.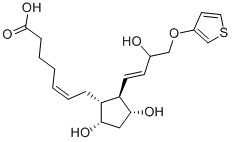
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Chemicals Category
Bimatoprost Chemical
Minimum Order Quantity : 1 Unit
Application : Pharmaceutical Industry
CAS No : 155206001
Grade : Medicine Grade
Form : Powder
8-Quinolinesulfonyl chloride
Minimum Order Quantity : 1 Ton
Application : Pharmaceutical Industry
CAS No : 18704375
Grade : Medicine Grade
Form : Solid
Storage : Room Temperature
4- methylsulfanyl(morpholin-4-yl)phosphoryl morpholine
Minimum Order Quantity : 1 Ton
Application : Pharmaceutical Industry
CAS No : 141930985
Form : Solid
Storage : Room Temperature
Alvimopan dihydrate
Minimum Order Quantity : 1 , , Ton
Application : Pharmaceutical Industry
CAS No : 170098381
Grade : Medicine Grade
Form : Powder
Storage : Room Temperature

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry