Call us now :+8613788933411
Tafluprost Chemical
Tafluprost Chemical Specification
- Molecular Formula
- C25H34F2O5
- Storage
- Freezer
- Place of Origin
- China
- Molecular Weight
- 452.53 Grams (g)
- Other Names
- 15,15-DIFLUORO-9ALPHA,11ALPHA-DIHYDROXY-16-PHENOXY-17,18,19,20-TETRANOR-PROSTA-5Z,13E-DIEN-1-OIC ACID, ISOPROPYL ESTER;AFP-168;TAFLUPROST
- CAS No
- 209860-87-7
- Grade
- Medicine Grade
- Usage
- Tafluprost is a prostaglandin analogue. It is used topically (as eye drops) to control the progression of open-angle glaucoma and in the management of ocular hypertension, alone or in combination with other medication. It reduces intraocular pressure by increasing the outflow of aqueous fluid from the eyes
- Purity
- 99%min
- Appearance
- white solid
- Application
- Pharmaceutical Industry
- Form
- Solid
Tafluprost Chemical Trade Information
- Minimum Order Quantity
- 1 Ton
- FOB Port
- Shanghai
- Payment Terms
- Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Paypal
- Supply Ability
- 1000 Tons Per Week
- Delivery Time
- 15 Days
- Sample Available
- Yes
- Sample Policy
- Contact us for information regarding our sample policy
- Packaging Details
- foil/bag/bottle
- Main Export Market(s)
- North America, South America, Eastern Europe, Western Europe, Middle East, Africa, Central America, Australia, Asia
About Tafluprost Chemical
Backed by rich industry experience, we are able to offer superior qualityTafluprost. Our qualified professionals precisely formulate these drugs from quality chemicals and other ingredients, sourced from the reliable vendors of the industry. These drugs are widely demanded for their effectiveness and non-allergic nature. The drugs we offer are quality examined by our expert members to ensure perfect TafluprostWe are able to deliver these products under a specific time-frame at market leading prices.
- CAS NO.: 209860-87-7
- Molecular Formula: C25H34F2O5
- Molecular Weight: 452.53
- Density: 1.186
Mechanism of Action
Tafluprost functions by mimicking the action of prostaglandins in the eye, which leads to increased outflow of aqueous humor. This mechanism effectively reduces intraocular pressurea key factor in treating glaucoma and ocular hypertension. By controlling pressure within the eye, Tafluprost aids in slowing or preventing further optic nerve damage.
Storage and Handling
This medication-grade chemical requires storage in a freezer to maintain stability and potency. Careful handling and adherence to pharmaceutical quality standards are critical for manufacturers and suppliers when distributing Tafluprost. Ensure the substance is kept in its solid form, away from direct heat or light.
Application in the Pharmaceutical Sector
Tafluprost is predominantly used in the development and production of eye drops for therapeutic application. It can be administered alone or in combination with other medications, giving flexibility in tailored treatment regimens for patients with elevated intraocular pressure. Its high purity and efficacy make it a preferred choice for pharmaceutical companies.
FAQs of Tafluprost Chemical:
Q: How is Tafluprost used in the treatment of eye conditions?
A: Tafluprost is administered topically as eye drops to lower intraocular pressure. It is used to manage open-angle glaucoma and ocular hypertension either as a stand-alone therapy or in combination with other medications.Q: What is the process by which Tafluprost reduces intraocular pressure?
A: Tafluprost increases the outflow of aqueous fluid from the anterior chamber of the eye, thus reducing intraocular pressure. This is achieved through its action as a prostaglandin analogue, mimicking natural prostaglandins to facilitate fluid movement.Q: When should Tafluprost be applied for optimal results?
A: Tafluprost is typically prescribed for daily use, often in the evening, but exact dosage and timing are determined by a physician. Regular and consistent application is important for effective management of eye pressure.Q: Where should Tafluprost be stored to ensure quality?
A: Tafluprost should be stored in a freezer as a solid to preserve its medicinal properties and prevent degradation. Proper storage is essential for maintaining its efficacy.Q: What are the primary benefits of using Tafluprost?
A: The main advantages include effective lowering of intraocular pressure, reduced risk of optic nerve damage, and flexibility of usealone or with other treatmentsmaking it highly valuable for patients with glaucoma or ocular hypertension.Q: How is the quality of Tafluprost maintained?
A: With a guaranteed purity of at least 99%, Tafluprost is produced under strict pharmaceutical-grade conditions. Quality control is maintained throughout the manufacturing, storage, and distribution processes.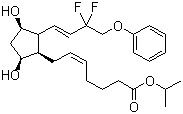
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Chemicals Category
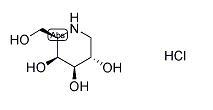
Migalastat hydrochloride
Minimum Order Quantity : 1 , , Ton
CAS No : 75172815
Other Names : DEOXYGALACTONOJIRIMYCIN, HYDROCHLORIDE
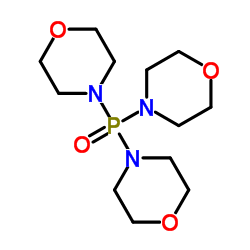
Trimorpholinophosphine Oxide
Minimum Order Quantity : 1 Ton
Storage : Room Temperature
Grade : Medicine Grade
CAS No : 4441127
Other Names : Trimorpholinophosphine Oxide
4-Cyanophenacyl Bromide
Minimum Order Quantity : 1 Ton
Storage : Room Temperature
Grade : Medicine Grade
CAS No : 20099892
Application : Pharmaceutical Industry
Other Names : 4Cyanophenacyl Bromide
vorapaxar sulfate M-9
Minimum Order Quantity : 1 , , Ton
Storage : Room Temperature
Grade : Medicine Grade
CAS No : 900161117
Application : Pharmaceutical Industry

 Send Inquiry
Send Inquiry




 Send Inquiry
Send Inquiry