Call us now :+8613788933411
Lubiprostone Chemicals
Lubiprostone Chemicals Specification
- Molecular Weight
- 390.46 Grams (g)
- Molecular Formula
- C20H32F2O5
- Other Names
- Lubiprostone Chemicals
- CAS No
- 136790-76-6
- Grade
- Medicine Grade
- Application
- Pharmaceutical Industry
Lubiprostone Chemicals Trade Information
- Minimum Order Quantity
- 1 Ton
- Supply Ability
- 1000 Tons Per Week
- Delivery Time
- 10 Days
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
About Lubiprostone Chemicals
Owing to our vast years of experience, we are manufacturing, supplying and exporting high-grade Lubiprostone. The compounds we offer are highly demanded in the market for its medical use and for formulating other drugs & medicines. Our vendors offer us with quality chemicals and ingredients to formulate these drugs. These drugs are mainly used for chronic idiopathic constipation and irritable bowel syndrome. Known for its effectiveness and non-allergic nature, the Lubiprostone is highly appreciated by our customers.
Other details:
- CAS NO. : 136790-76-6
- Molecular Weight: 390.46
- Molecular Formula: C20H32F2O5
Applications in the Pharmaceutical Industry
Lubiprostone Chemicals play a crucial role as an active pharmaceutical ingredient, widely utilized in medication development and production. Their consistent quality supports regulatory compliance and efficacy in final products for various therapeutic uses. Medicine-grade standards ensure that these chemicals meet strict purity requirements, making them suitable for further processing and formulation within pharmaceutical operations.
Sourcing and Supply Chain
Experienced suppliers, exporters, and manufacturers in China manage the distribution of Lubiprostone Chemicals globally. Reliable supply chains, established by these entities, guarantee timely delivery and adherence to international standards. Traders ensure product availability for pharmaceutical companies, research institutions, and other end-users, streamlining the procurement process and providing technical documentation as required.
FAQs of Lubiprostone Chemicals:
Q: How are Lubiprostone Chemicals typically used in the pharmaceutical industry?
A: Lubiprostone Chemicals are primarily used as pharmaceutical-grade active ingredients in the formulation of medications that address gastrointestinal disorders. Their application focuses on supporting therapeutic solutions that enhance intestinal fluid secretion and motility.Q: What are the benefits of using medicine-grade Lubiprostone Chemicals?
A: The main benefit of medicine-grade Lubiprostone is its high purity and conformance to pharmaceutical standards. This ensures reliable performance in drug development processes and mitigates risk related to impurities, safeguarding final product quality and patient safety.Q: When should companies consider sourcing Lubiprostone Chemicals?
A: Pharmaceutical manufacturers and research organizations should consider sourcing Lubiprostone Chemicals during the pre-formulation and formulation phases of drug development, or when there is a requirement for high-purity ingredients in approved medication production.Q: Where are Lubiprostone Chemicals distributed from?
A: Lubiprostone Chemicals are distributed, exported, and supplied mainly from China by authorized distributors, exporters, and manufacturers who provide global coverage and technical support for international buyers.Q: What is the standard process for procuring Lubiprostone Chemicals?
A: The procurement process usually involves contacting verified suppliers or manufacturers, reviewing technical specifications and compliance documents, and then arranging supply agreements and logistics for delivery to the required location.Q: How should Lubiprostone Chemicals be stored and handled?
A: These chemicals should be stored in a cool, dry place away from direct sunlight and moisture. Proper handling and storage conditions are essential to maintain product integrity and meet pharmaceutical quality requirements.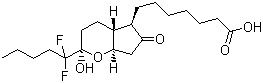
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Chemicals Category
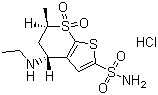
Dorzolamide Hydrochloride
Minimum Order Quantity : 1 Ton
Application : Pharmaceutical Industry
CAS No : 120279961
Grade : Medicine Grade
Usage : Dorzolamide hydrochloride is used to lower increased intraocular pressure in openangle glaucoma and ocular hypertension.
Storage : Freezer
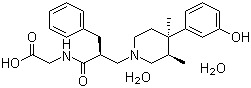
Alvimopan dihydrate
Minimum Order Quantity : 1 , , Ton
Application : Pharmaceutical Industry
CAS No : 170098381
Grade : Medicine Grade
Usage : Industrial
Storage : Room Temperature
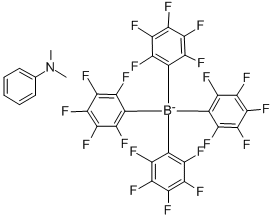
N N Dimethylanilinium tetrakis pentafluorophenyl borate
Minimum Order Quantity : 1 Ton
Application : Pharmaceutical Industry
CAS No : 118612003
Grade : Medicine Grade
Storage : Other
4-Fluoroisoquinoline-5-sulfonyl chloride
Minimum Order Quantity : 1 Ton
Application : Pharmaceutical Industry
CAS No : 194032332
Usage : intermediate

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry