Call us now :+8613788933411
Eliglustat chemical
Eliglustat chemical Specification
- Molecular Weight
- 404.54294 Drams (dr)
- Molecular Formula
- C23H36N2O4
- Place of Origin
- China
- Other Names
- Eliglustat;Genz 99067;N-[(1R,2R)-2-(2,3-Dihydro-1,4-benzodioxin-6-yl)-2-hydroxy-1-(1-pyrrolidinylmethyl)ethyl]-octanamide;N-[(1R,2R)-1-(2,3-Dihydro-1,4-benzodioxin-6-yl)-1-hydroxy-3-(1- pyrrolidinyl)-2-propanyl]octanamide;Eliglustat(Genz-99067)
- CAS No
- 491833-29-5
- Usage
- Eliglustat is a glucosylceramide synthase inhibitor indicated for the long-term treatment of type 1 Gaucher disease. Patients selected for treatment with Eliglustat undergo an FDA approved genotype
- Purity
- 99%
- Smell
- Other
- Color
- Other
- Form
- Powder
Eliglustat chemical Trade Information
- Minimum Order Quantity
- 1 Ton
- FOB Port
- Shanghai
- Payment Terms
- Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Cash Advance (CA)
- Supply Ability
- 1000 Tons Per Week
- Delivery Time
- 15 Days
- Sample Available
- Yes
- Sample Policy
- Contact us for information regarding our sample policy
- Packaging Details
- foil bag/ plastic bag/bottles
- Main Export Market(s)
- Australia, North America, Eastern Europe, Middle East, Africa, Western Europe, Central America, South America, Asia
About Eliglustat chemical
We have established ourselves as a renowned manufacturer, exporter and supplier ofEliglustatOur skilled professionals formulate these compounds by making use of various ingredients and chemicals available in the market. We formulate these drugs in our using latest equipmentthereforethese medicines havelongershelf life and are very effective. TheEliglustatis available in various specifications to meet our clients demands.
details:
- Product name:Eliglustat
- Synonyms:benzodioxin-6-yl)-2-hydroxy-1-(1-pyrrolidinylmethyl)ethyl]-octanamide;N-[(1R,2R)-1-(2,3-Dihydro-1,4-benzodioxin-6-yl)-1-hydroxy-3-(1- pyrrolidinyl)-2-propanyl]octanamide;Eliglustat(Genz-99067)
- CAS NO.: 491833-29-5
- Molecular Formula: C23H36N2O4
- Molecular Weight: 404.54294
Trusted Treatment for Type 1 Gaucher Disease
Eliglustat stands out as an effective oral medication selected for long-term management of type 1 Gaucher disease. Its targeted action helps reduce the burden of harmful lipid accumulation by inhibiting glucosylceramide synthase. Patients benefit from improved quality of life, with reduced symptoms and disease progression.
Exceptional Purity and Quality Assurance
Produced in China, Eliglustat boasts a high purity level of 99%, making it ideal for pharmaceutical use. Stringent quality controls and advanced manufacturing processes ensure the consistent reliability and efficacy of each batch, meeting international standards for therapeutic agents.
Comprehensive Supplier Network
As a widely sought-after pharmaceutical ingredient, Eliglustat is distributed globally through reputable exporters, traders, and suppliers based in China. This broad distribution network ensures timely availability and delivery, supporting ongoing therapeutic programs worldwide.
FAQs of Eliglustat chemical:
Q: How is Eliglustat used in the treatment of type 1 Gaucher disease?
A: Eliglustat is administered orally and works by inhibiting the enzyme glucosylceramide synthase, thus reducing the buildup of harmful lipids within cells. This helps manage and alleviate symptoms associated with type 1 Gaucher disease over long-term use.Q: What is the process for selecting patients for Eliglustat therapy?
A: Patients are selected for Eliglustat treatment based on results from an FDA-approved genotype test. This genetic screening ensures that only individuals who are likely to metabolize the medication safely and effectively are prescribed the therapy.Q: When should Eliglustat be considered for type 1 Gaucher disease management?
A: Eliglustat is typically considered for patients diagnosed with type 1 Gaucher disease when long-term therapy is indicated and the patients genotype has been confirmed as suitable. This decision is made in consultation with a healthcare professional.Q: Where is Eliglustat manufactured and distributed from?
A: Eliglustat is produced in China and is distributed internationally by various reputable exporters, distributors, manufacturers, suppliers, and traders. This ensures global access to the pharmaceutical compound.Q: What benefits does Eliglustat offer compared to other treatments?
A: Eliglustat offers the convenience of oral administration and targets the underlying cause of lipid accumulation in type 1 Gaucher disease. With high purity and reliable therapeutic effect, it can improve clinical symptoms and overall patient outcomes.Q: What is the purity and chemical composition of Eliglustat?
A: Eliglustat is supplied in powder form with a certified purity of 99%. It has the molecular formula C23H36N2O4 and a CAS number 491833-29-5, ensuring pharmaceutical-grade quality for clinical use.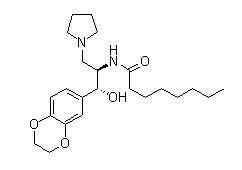

Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Chemicals Category
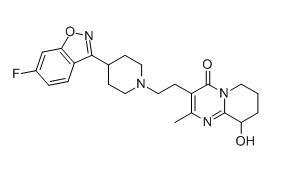
Paliperidone chemical
Minimum Order Quantity : 1 Ton
CAS No : 144598754
Molecular Weight : 426.48 Milligram (mg)
Molecular Formula : C23H27FN4O3
Usage : Paliperidone is mainly used to treat schizophrenia and schizoaffective disorder. It is also used to treat mania and at lower doses as maintenance for bipolar disorder.
Other Names : 4hpyrido(2,1a)pyrimidin4one,6,7,8,9tetrahydro3(2(4(6fluro1,2benzis ;oxazol3yl)1piperidinyl)ethyl)9hydroxy2methyl ;r76477 ;9HYDROXYRISPERIDONE;Paliperidone;rac9Hydroxyrisperidone;6,7,8,9Tetrahydro3(2(4(6fluro1,2benzisoxazol3yl)1piperidinyl)ethyl)9hydroxy2methyl4Hpyrido[2,1a]pyrimidin4one;Paliperidone for research
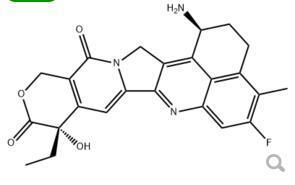
Exatecan 171335-80-1
Minimum Order Quantity : 1 Ton
CAS No : 171335801
Molecular Weight : 435.45 Grams (g)
Molecular Formula : C24H22FN3O4
Usage : API
Other Names : Exatecan
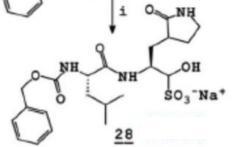
GC 376
Minimum Order Quantity : 1 Ton
CAS No : 1416992396
Molecular Weight : 507.5338 Ounce (oz)
Molecular Formula : C21H30N3NaO8S
Usage : API
Other Names : sodium (2S)2((S)2(((benzyloxy)carbonyl)amino)4methylpentanamido)1hydroxy3 (2oxopyrrolidin3yl)propane1sulfonate
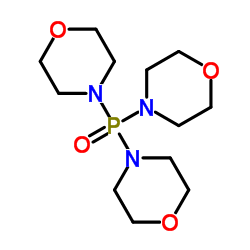
Trimorpholinophosphine Oxide
Minimum Order Quantity : 1 Ton
CAS No : 4441127
Molecular Weight : 305.310 Grams (g)
Molecular Formula : C12H24N3O4P
Other Names : Trimorpholinophosphine Oxide

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry