Call us now :+8613788933411
Cholecalciferol Impurity A 5 6 trans Cholecalciferol 5 6 trans Vitamin D3
Cholecalciferol Impurity A 5 6 trans Cholecalciferol 5 6 trans Vitamin D3 Specification
- Storage
- Room Temperature
- CAS No
- 22350-41-0
- Grade
- Medicine Grade
- Application
- Pharmaceutical Industry
- Form
- Solid
Cholecalciferol Impurity A 5 6 trans Cholecalciferol 5 6 trans Vitamin D3 Trade Information
- Minimum Order Quantity
- 1 Ton
- Supply Ability
- 1000 Tons Per Week
- Delivery Time
- 15 Days
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Africa
About Cholecalciferol Impurity A 5 6 trans Cholecalciferol 5 6 trans Vitamin D3
Cholecalciferol Impurity A 56transCholecalciferol 56transVitamin D3
Technical Specification
Product Name Cholecalciferol Impurity A 56transCholecalciferol 56transVitamin D3
MF C27H44O
MW 38464
CAS 22350410
Ensuring Pharmaceutical Quality
Cholecalciferol Impurity A is critical for maintaining high standards in drug manufacturing. Its precise composition helps detect and quantify impurities in Vitamin D3 medicines, enabling pharmaceutical companies to adhere to international quality regulations and guarantee patient safety.
Versatile Role in Research and Development
This impurity standard aids researchers in studying Vitamin D3 formulation stability and degradation pathways. Its availability as a solid, medicine-grade product makes it compatible with various analytical techniques, supporting robust product development and innovation.
Trusted Supply and Distribution from China
As a product offered by distributors, exporters, manufacturers, suppliers, and traders in China, Cholecalciferol Impurity A benefits from a reliable supply chain. Stringent manufacturing practices ensure consistency, while accessible distribution channels meet both domestic and global pharmaceutical needs.
FAQs of Cholecalciferol Impurity A 5 6 trans Cholecalciferol 5 6 trans Vitamin D3:
Q: How is Cholecalciferol Impurity A used in the pharmaceutical industry?
A: Cholecalciferol Impurity A is primarily used as a reference standard in quality control and research laboratories for the analysis of Vitamin D3 formulations. It helps in identifying and quantifying impurities to ensure pharmaceutical products meet regulatory standards.Q: What benefits does 5,6-trans Cholecalciferol offer in medicine manufacturing?
A: This impurity standard supports accurate detection and quantification of possible by-products in Vitamin D3 medications. Its use improves product safety, regulatory compliance, and data reliability during pharmaceutical production and analysis.Q: When should Cholecalciferol Impurity A be incorporated into pharmaceutical processes?
A: This impurity is typically employed during formulation development, stability studies, method validation, and routine quality assurance testing, particularly when monitoring the purity of Vitamin D3 medications.Q: Where is Cholecalciferol Impurity A produced and supplied from?
A: Cholecalciferol Impurity A is widely manufactured, exported, and supplied by companies in China, who ensure quality compliance and support global pharmaceutical supply chains.Q: What is the recommended storage condition for this impurity standard?
A: Cholecalciferol Impurity A should be stored at room temperature in its solid form to maintain its stability and integrity for accurate laboratory analyses.Q: Can you describe the process for using this impurity in laboratory analysis?
A: The impurity is typically dissolved or dispersed in suitable solvents according to analytical protocols. It is then analyzed alongside pharmaceutical samples using advanced techniques, such as HPLC, to quantify and monitor impurity levels.Q: Why is Cholecalciferol Impurity A important for pharmaceutical quality control?
A: It plays a key role in ensuring that Vitamin D3 medicines are free from critical impurities, thus upholding product quality, supporting approvals, and safeguarding patient health.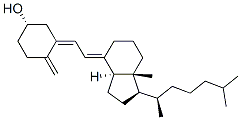
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Chemicals Category
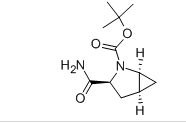
(1S 3S 5S)-3-(AMinocarbonyl)-2-azabicylo 3.1.0 hexane-2-carboxylic acid tert-butyl ester
Minimum Order Quantity : 1 Ton
Application : Pharmaceutical Industry
Grade : Medicine Grade
Usage : Intermediate
Storage : Dry Place
CAS No : 361440677
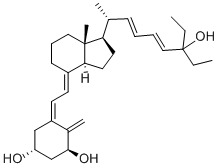
Seocalcitol Chemical
Minimum Order Quantity : 1 Ton
Application : Pharmaceutical Industry
Grade : Medicine Grade
Usage : Seocalcitol is a Vitamin D receptor (VDR) agonist; Seocalcitol is a analog of calcitriol.Seocalcitol exhibits antitumor and antiproliferative activity with reduced hypercalcemic effects.
Storage : Freezer
CAS No : 134404527
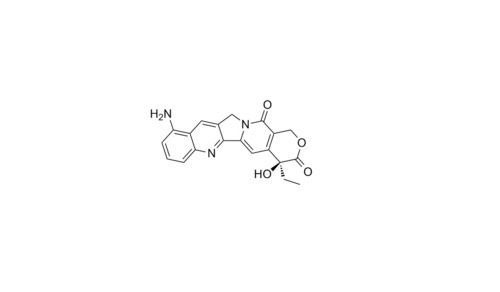
9-Aminocamptothecin API
Minimum Order Quantity : 1 Ton
Application : Pharmaceutical Industry
Grade : Medicine Grade
Storage : Room Temperature
CAS No : 91421431
1R 2R-1 2-Diamino-1 2-diphenylethane
Minimum Order Quantity : 1 Ton
Application : Pharmaceutical Industry
Grade : Medicine Grade
Usage : Industrial
Storage : Room Temperature
CAS No : 35132208

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry