Call us now :+8613788933411
Carboprost Chemical
Carboprost Chemical Specification
- Molecular Weight
- 368.51 Grams (g)
- Storage
- Freezer
- Molecular Formula
- C21H36O5
- Other Names
- 5z,9-alpha,11-alpha,13e,15s)-9,11,15-trihydroxy-15-methylprosta-5,13-dien-1;15(s)-15-methylpgf2-alpha
- CAS No
- 35700-23-3
- Grade
- Medicine Grade
- Usage
- Carboprost induces contractions and can trigger abortion in early pregnancy. It also reduces postpartum bleeding.
- Purity
- 98%min
- Appearance
- white solid
- Application
- Pharmaceutical Industry
- Color
- White
- Form
- Solid
Carboprost Chemical Trade Information
- Minimum Order Quantity
- 1 Ton
- FOB Port
- Shanghai
- Payment Terms
- Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Paypal
- Supply Ability
- 1000 Tons Per Week
- Delivery Time
- 15 Days
- Sample Available
- Yes
- Sample Policy
- Contact us for information regarding our sample policy
- Packaging Details
- foil/bag/bottle
- Main Export Market(s)
- Australia, Eastern Europe, Africa, Central America, Middle East, South America, Western Europe, Asia, North America
About Carboprost Chemical
Backed by rich industry experience, we are offer our customers with Carboprost. These compounds are used for aborting in early pregnancy. We formulate these medicines keeping in mind the industry laid parameters and the well-being of our valued customers. Our vendors provide us with the required chemicals and ingredients to formulate these drugs. All our medicines are examined and tested before deliver to offer our customers with effective and non-allergic Carboprost.
Other details:
- CAS NO.: 35700-23-3
- Molecular Formula: C21H36O5
- Molecular Weight: 368.51
Pharmaceutical Applications of Carboprost
Carboprost, renowned for its role in the pharmaceutical sector, is primarily employed to induce uterine contractions. It is a critical agent in safely terminating early pregnancies and minimizing postpartum hemorrhage, ensuring maternal health outcomes are optimized. Its high purity guarantees consistent performance when formulated with precision by healthcare manufacturers.
Optimal Storage and Handling Guidelines
To preserve the integrity of Carboprost, it should be kept in a freezer away from direct light and moisture. Proper storage ensures the compound retains its solid white form and desired chemical properties, minimizing the risk of degradation or reduced effectiveness during distribution and use.
Safety and Usage Precautions
Carboprost is a potent compound and must be handled by trained professionals in controlled environments. Strict adherence to dosage and application protocols in the pharmaceutical industry protects both patients and practitioners, and its usage is strictly regulated for safety and efficacy reasons.
FAQs of Carboprost Chemical:
Q: How should Carboprost be stored to maintain its quality?
A: Carboprost requires freezer storage to ensure it retains its stability and effectiveness. Keeping the compound at low temperatures preserves its solid white appearance and prevents chemical degradation, making it suitable for pharmaceutical applications.Q: What is the primary use of Carboprost in medicine?
A: Carboprost is chiefly used to induce uterine contractions, specifically for triggering abortion in early pregnancy and controlling excessive postpartum bleeding. Its effectiveness in these roles makes it a vital component in maternal healthcare management.Q: When is Carboprost typically administered in clinical settings?
A: Carboprost is administered when there is a medical necessity for early pregnancy termination or to manage severe postpartum hemorrhage, under the supervision of healthcare professionals to ensure proper dosage and minimize risks.Q: Where does the supply and manufacture of Carboprost generally take place?
A: Carboprost is widely manufactured and distributed from China, where it is supplied to various pharmaceutical entities, including exporters, distributors, and traders for global medical use.Q: What processes are involved in utilizing Carboprost within the pharmaceutical industry?
A: Carboprost undergoes careful formulation and quality control to ensure it meets medicine-grade standards. Its solid form allows precise dosing, and it is incorporated into medical protocols regulated by healthcare authorities.Q: What benefits does Carboprost offer in medical treatments?
A: The key benefit of Carboprost is its ability to induce strong uterine contractions, which aids in the safe management of early-term abortion and the prevention of life-threatening postpartum hemorrhage, contributing to improved patient outcomes.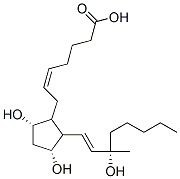
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Chemicals Category
Alfacalcidol chemical
Minimum Order Quantity : 1 Ton
Grade : Medicine Grade
Application : Pharmaceutical Industry
CAS No : 41294568
Storage : Freezer
Molecular Weight : 400.64 Grams (g)
Paliperidone chemical
Minimum Order Quantity : 1 Ton
Grade : Medicine Grade
CAS No : 144598754
Molecular Weight : 426.48 Milligram (mg)
Glatiramer acetate
Minimum Order Quantity : 1 , , Ton
Grade : Medicine Grade
Application : Pharmaceutical Industry
CAS No : 147245929
Storage : Room Temperature
Molecular Weight : 623.65 GSM (gm/2)
Vorapaxar Sulfate
Minimum Order Quantity : 1 Ton
Grade : Medicine Grade
Application : Pharmaceutical Industry
CAS No : 705260088
Storage : Room Temperature

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry