Call us now :+8613788933411
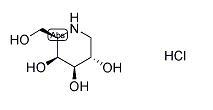
Calcifediol Monohydrate
Calcifediol Monohydrate Specification
- Molecular Weight
- 400.64 Grams (g)
- Molecular Formula
- C27H44O2
- Place of Origin
- China
- Other Names
- CALCIDIOL;CALCIFEDIOL;CALCIFEDIOL MONOHYDRATE;25-OH-D3;25-HYDROXYVITAMIN D
- CAS No
- 63283-36-3
- Grade
- Medicine Grade
- Usage
- Calcifediol is then converted in the kidneys (by the enzyme 25(OH)D-1-hydroxylase) into calcitriol (1,25-(OH)2D3), a secosteroid hormone that is the active form of vitamin D. It can also be converted into 24-hydroxycalcidiol in the kidneys via 24-hydroxylation.
- Purity
- 98%min
- Appearance
- white solid
- Application
- Pharmaceutical Industry
- Form
- Powder
Calcifediol Monohydrate Trade Information
- Minimum Order Quantity
- 1 Ton
- FOB Port
- Shanghai
- Payment Terms
- Letter of Credit (L/C), Paypal, Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T)
- Supply Ability
- 1000 Tons Per Week
- Delivery Time
- 10 Days
- Sample Available
- Yes
- Sample Policy
- Contact us for information regarding our sample policy
- Packaging Details
- Foil/bag/bottle
- Main Export Market(s)
- North America, Eastern Europe, Western Europe, Africa, Central America, Australia, Middle East, South America, Asia
About Calcifediol Monohydrate
We are a coveted organization engaged in offering our valuable clients with Calcifediol . These chemicals are mostly demanded as an ingredient for cosmetics and for reducing fat. We formulate these drugs from quality chemicals and other ingredients brought from the renowned vendors of the market. Being effective and non-allergic, these products are widely demanded in the market. These compounds are quality examined before delivery to offer our clients with the best Calcifediol .
Product Details
- CAS NO.: 63283-36-3
- Molecular Formula: C27H44O2
- Molecular Weight: 400.64
Key Pharmaceutical Applications
Calcifediol Monohydrate is vital in formulating medications used to address vitamin D deficiency and related metabolic disorders. Its reliable activity profile ensures its effectiveness in clinical and research settings, making it a preferred ingredient by pharmaceutical manufacturers worldwide.
High Purity for Superior Performance
Produced with a minimum purity of 98%, this medicine-grade compound meets stringent industry standards. Its high quality ensures consistent results and safety for pharmaceutical development, guaranteeing the effectiveness of vitamin D therapies.
Expert Manufacturing and Global Distribution
Manufactured in China, Calcifediol Monohydrate is widely distributed by leading exporters, suppliers, and traders. Its accessibility makes it a staple for pharmaceutical companies looking for reliable sources of key vitamin D precursors.
FAQs of Calcifediol Monohydrate:
Q: How is Calcifediol Monohydrate utilized in the pharmaceutical industry?
A: Calcifediol Monohydrate is primarily used as an intermediate in pharmaceutical preparations to support or investigate vitamin D metabolism. It is employed in medications that treat or prevent vitamin D deficiency by serving as a precursor to the active hormone calcitriol.Q: What is the process by which Calcifediol Monohydrate is transformed into its active form in the body?
A: Once administered, Calcifediol Monohydrate undergoes enzymatic conversion in the kidneys by 25(OH)D-1-hydroxylase, resulting in calcitriol, the physiologically active form of vitamin D that regulates calcium and phosphate homeostasis.Q: When should Calcifediol-based pharmaceuticals be considered for use?
A: Pharmaceuticals containing Calcifediol Monohydrate are recommended for individuals with vitamin D deficiency or metabolic bone diseases, typically when cutaneous synthesis or absorption of vitamin D is insufficient.Q: Where is Calcifediol Monohydrate manufactured and supplied from?
A: This compound is produced in China by established manufacturers and is distributed globally by various exporters, suppliers, and traders to meet pharmaceutical industry demands.Q: What are the main benefits of using Calcifediol Monohydrate in medication formulations?
A: Using Calcifediol Monohydrate offers reliable conversion to active vitamin D, supporting bone and mineral health, and providing a standardized approach for treating deficiencies in clinical settings.Q: How does the purity of Calcifediol Monohydrate impact its pharmaceutical effectiveness?
A: A minimum purity of 98% ensures that Calcifediol Monohydrate delivers consistent results in medical applications, minimizing impurities and maximizing safety for patient care.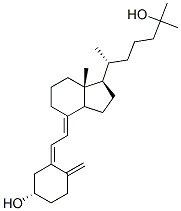
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Chemicals Category
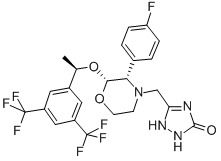
Aprepitant Chemical
Minimum Order Quantity : 1 Ton
Usage : Aprepitant is useful in the treatment of cyclic vomiting syndrome and latestage chemotherapy induced vomiting, but there are few studies to date. On January 2008, the FDA approved fosaprepitant, an intravenous form of aprepitant.
CAS No : 170729803
Other Names : 5[2(r)[1(r)[3,5bis(trifluoromethyl)phenyl]ethoxy]3(s)(4fluorophenyl)morpholin4ylmethyl]3,4dihydro2h1,2,4triazol3one;aprepitant
Purity : 99%min
Molecular Weight : 534.427 Grams (g)
Eribulin chemical
Minimum Order Quantity : 1 Ton
Usage : It is used to treat certain patients with breast cancer and liposarcoma.
CAS No : 253128415
Other Names : B 1939; E 7389; ER 086526
Purity : 99%
Molecular Weight : C40H59NO11 Drams (dr)
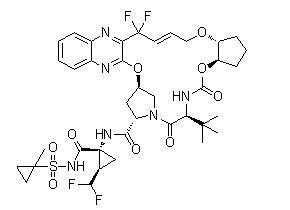
Glecaprevir Chemical
Minimum Order Quantity : 1 Ton
Usage : Glecaprevir is a hepatitis C virus (HCV) nonstructural (NS) protein 3/4A protease inhibitor that was identified jointly. It is being developed as a treatment of chronic hepatitis C infection in cofor
CAS No : 1365970031
Other Names : (1R,2R)N[[[(1R,2R)2[[4,4Difluoro4(3hydroxy2quinoxalinyl)2buten1yl]oxy]cyclopentyl]oxy]carbonyl]3methylLvalyl(4R)4hydroxyLprolyl1amino2(difluoromethyl)N[(1methylcyclopropyl)sulfonyl]cyclopropanecarboxamide cyclic (12)ether; A 1282576.0; ABT 493
Purity : 99%
Molecular Weight : 838.87 Grams (g)
Migalastat hydrochloride
Minimum Order Quantity : 1 , , Ton
Usage : it is a drug for the treatment of Fabry disease, a rare genetic disorder.
CAS No : 75172815
Other Names : DEOXYGALACTONOJIRIMYCIN, HYDROCHLORIDE
Purity : 99%
Molecular Weight : 199.63 Milligram (mg)

 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry